Submucosal Injection Agent
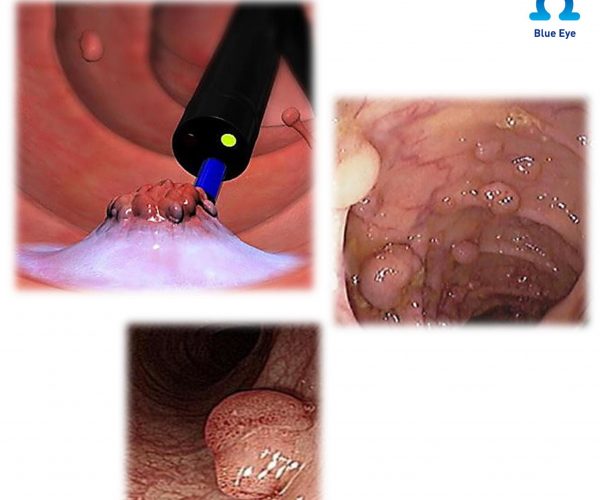
Blue Eye™ is a medical device that is an injectable liquid composition intended for use in gastrointestinal
endoscopic procedures for submucosal lift of polyps, adenomas, early stage cancers or other
gastrointestinal mucosal lesions, prior to removal with a snare or endoscopic device.
• It facilitates endoscopic resection procedures during endoscopic examinations in the upper and lower
gastrointestinal tract, such as the esophagus, the stomach.
1. Blue Eye™ is easy to use.
Unlike conventional ampoule products, Blue Eye™ is easy to use because it can be connected directly with the injection needle.
2. Blue Eye™ is safe from contamination.
Blue Eye™ is packaged in a ready-to-use syringe with steam-sterilized solution that connects directly to the injection needle with a Luer-lock
to eliminate the risk of cross-contamination by preventing air exposure.
3. Blue Eye™ is effective.
Blue Eye™ has twice longer-lasting mucosal elevation than normal saline, which reduces entire procedure time and side effects such as
perforation, which increases the success rate of the endoscopic procedure.
4. Blue Eye™ is biocompatible.
Blue Eye™ is biocompatible; it contains hyaluronic acid, a biopolymer naturally present in the human dermis.
Important Safety Information
▶ WARNINGS AND PRECAUTIONS
• The safety of Blue Eye™ has not been established in pregnant or lactating women, or in children under 18 years of age.
• The endoscopist injecting Blue Eye™ must be experienced in the administration technique.
▶ ADVERSE REACTIONS
• Rarely, local bleeding and/or inflammatory reaction could occur which may or may not be associated with Blue Eye™.
▶ CONTRADICTIONS
• Patients with known sensitivity to any of the components contained in Blue Eye™. Please see Instructions for Use for complete Important
Safety Information.